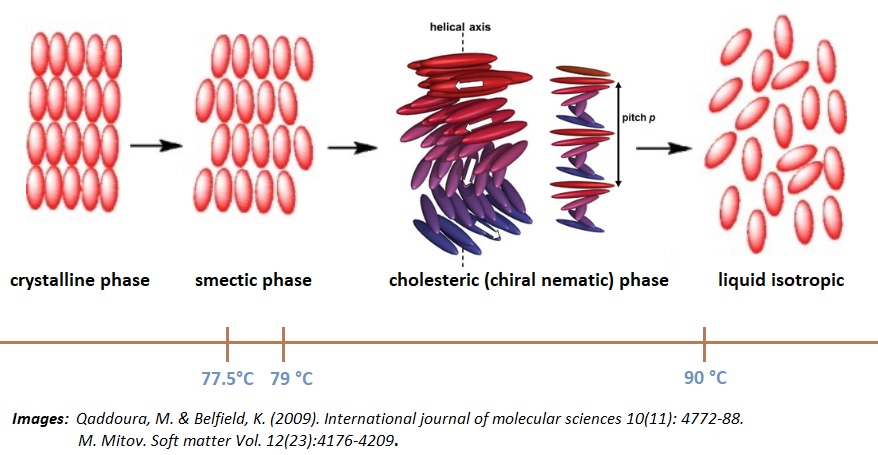
| phases | temperature range | type of phase |
| crystalline | up to 77.5 °C | anisotropic |
| smectic | 77.5 °C - 79 °C | anisotropic |
| cholesteric (chiral nematic) | 79 °C - 90 °C | anisotropic |
| isotropic | greater than 90 °C | isotropic |
More details on Cholesteryl Pelargonate 97% we use.
This self-made video shows Cholesteryl Pelargonate 97% between two polarisation filters. At the beginning it is liquid. As it cools it undergoes different phases. While passing the chiral nematic phase we see the appearing rainbow colors. As the temperature of the LC cools further it crystallizes and becomes birefingent and therefore lets light pass between crossed polarisation. Birefringence and dichroism are a typical feature of liquid crystals.
Temperature dependent reflection of Cholesteryl Pelargonate 97%
Home
Within the cholesteric (chiral nematic) phase of Cholesteryl Pelargonate 97%, the molecular structure order in a chiral nematic manner. When the twist of one chiral revolution coincides with the wavelengh of incident light, this wavelength is reflected and we se a particular color at a certain temperature. With temperture thes twists are shorter and the reflection gets "bluer". In the picture below this temperature dependent color reflection is shown together with the liquid and solid phase.

Angle dependent reflection of Cholesteryl Pelargonate 97%
Home
 The sample is in its chiral nematic phase at a temperature of ~ 91 °C. The incidence angle of the light is at 33° relative to the front side. Left circularly polarized is used because it produces the highest reflection of colors. With right circularly polarized light the reflection disappears and the "whight light" is transmitted through the sample.
We see different colors reflecting at different angles due to the wavelength dependent refraction index (dichroism).
The sample is in its chiral nematic phase at a temperature of ~ 91 °C. The incidence angle of the light is at 33° relative to the front side. Left circularly polarized is used because it produces the highest reflection of colors. With right circularly polarized light the reflection disappears and the "whight light" is transmitted through the sample.
We see different colors reflecting at different angles due to the wavelength dependent refraction index (dichroism).
Polarized optical microscopy (POM) on Cholesteryl Pelargonate 97%
Home
Figure 1 shows a POM image of the the Cholesteryl Pelargonate 97% in its solid state. The sample was
squeezed between two glass plates while still liquid with greater than 90°C. While cooling
it crystallized into spherulites.
These formations are due to molecular alignments as see in the inset of Fig. 2. They
crystallization starts from the center of he spherulite and continues outward in a
radial manner. The spherulites are in the order of up to one milimeter.
Fig. 2 shows a detailed view of some sherulites. These molecular alignments can be made visibly by placing the sample between crossed polarization filters and a magnifying objective, or microscope. This technique is called polarized optical microscopy (POM). Without crossed polarization one would only see homogeneous transparency.

Fig. 1: Spherulites crystallized from Cholesteryl Pelargonate between two glass plates made visible throught with crossed polarized light and a magnifying glass.

Fig. 2: Enlarged view of some spherulite of Cholesteryl Pelargonate made visible with a microscope a polarized light source and a crossed polarization analyzer (POM). The inset shows how the moleculs align in order to produce the light contrasts characteristic in spherulites.
Illustration on how spherulites are formed is shown in the video below. These formations form in crystalline polymers or liquid crystals. Both are optically birefringent and is the reson why these dark and bright shades are visible.
Last update: 4th of February, 2021
Fig. 2 shows a detailed view of some sherulites. These molecular alignments can be made visibly by placing the sample between crossed polarization filters and a magnifying objective, or microscope. This technique is called polarized optical microscopy (POM). Without crossed polarization one would only see homogeneous transparency.

Fig. 1: Spherulites crystallized from Cholesteryl Pelargonate between two glass plates made visible throught with crossed polarized light and a magnifying glass.

Fig. 2: Enlarged view of some spherulite of Cholesteryl Pelargonate made visible with a microscope a polarized light source and a crossed polarization analyzer (POM). The inset shows how the moleculs align in order to produce the light contrasts characteristic in spherulites.
Illustration on how spherulites are formed is shown in the video below. These formations form in crystalline polymers or liquid crystals. Both are optically birefringent and is the reson why these dark and bright shades are visible.
Last update: 4th of February, 2021
